Weather: Under Pressure
The force pushing down on you

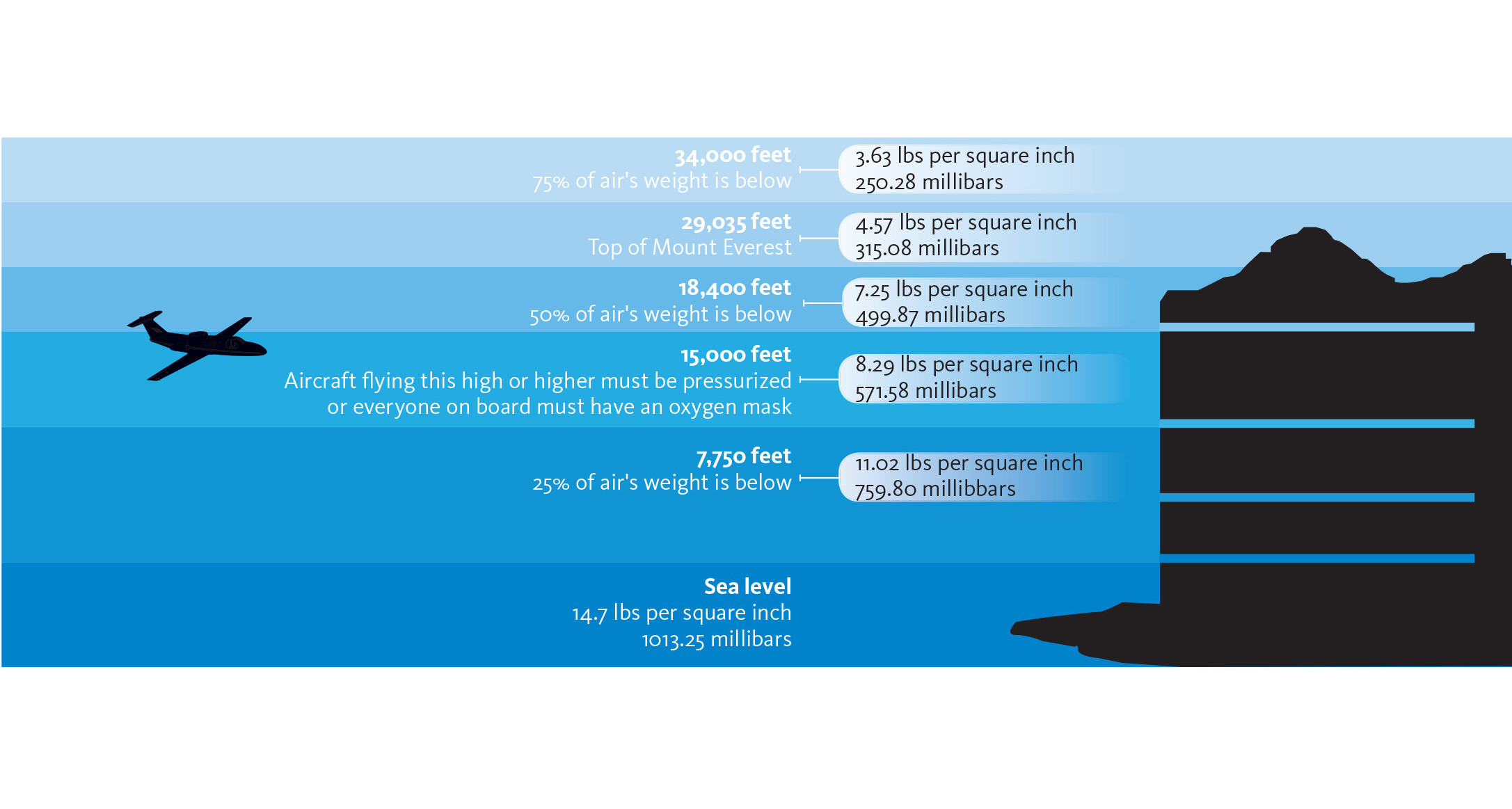
Standard sea level pressure is 29.92 inches Hg. or 1013 millibars.
Most people go through life without knowing that the air is exerting a pressure of approximately 14.7 pounds per square inch on them and everything around them when close to sea level. Pilots, on the other hand, have good reasons to understand the basics of atmospheric pressure.
Air is an invisible gas, but it’s no less real just because we can’t see it. The gases that make up the air have mass.
About 78 percent of Earth’s air is nitrogen; about 21 percent is oxygen; and the remaining 1 percent is a host of other gases, including water vapor. In this article, air molecules refers to all of the molecules—nitrogen, oxygen, water vapor, and so on—that make up air.
The balance between gravity and molecular motion. Since air molecules have mass, gravity pulls them toward the center of the Earth. So what keeps them from piling up on the ground like sand dumped from a truck?
They don’t pile up because they are zipping around at high speeds, colliding with other air molecules and anything else in the air—such as you and your airplane. As gravity pulls air molecules down, collisions with upward-moving air molecules push them back up.
The pressure of the air at any altitude depends on the weight of the air above that’s pushing down on it. Since gases such as air are compressible, applying pressure to them increases their density. The result is that air becomes less dense and its pressure decreases with altitude.
 A microscopic look at air. If you could somehow see the billions of air molecules in a jar, you’d see that the jar is mostly empty. In fact, the air around you is about 99.9 percent empty space.
A microscopic look at air. If you could somehow see the billions of air molecules in a jar, you’d see that the jar is mostly empty. In fact, the air around you is about 99.9 percent empty space.
How can so relatively few air molecules apply so much pressure?
Air molecules near the Earth’s surface are moving, on the average, approximately 1,000 mph. One cubic centimeter of air—roughly the size of the tip of your little finger—contains a huge number of molecules. If you wrote it down it would be a one followed by 20 zeros.
The extremely large number of tiny molecules moving at such high speeds creates pressure.
Since there is so much empty space between the molecules of a gas, any kind of force can easily compress a gas. Liquids and solids, which don’t have nearly as much room between their molecules, are extremely hard to compress.
The importance of air density. Understanding the air’s density is necessary in order to make sense of how the atmosphere and aircraft work. Density is a measure of how much mass of a substance a particular volume contains. In general, the higher the air’s density, the better aircraft perform.
At sea level, the air’s average density is 1.225 kilograms per cubic meter. In units commonly used in the United States, we would say that a cubic foot of air weighs 0.077 pounds.
Air’s density changes with variations in atmospheric pressure, such as when a storm’s low atmospheric pressure center arrives, and also with changes in temperature. If the air’s pressure stays the same, the air becomes denser as the temperature drops.
You often hear about cold air being denser than warm air. This can be confusing to someone who is just beginning to learn about weather. “If cold air is denser than warm air,” the beginner might ask, “and the air aloft is usually colder than the air at the surface, why doesn’t the cold air aloft sink?” The answer is that lower atmospheric pressure and the resulting lower density aloft more than make up for the air being colder than the air below.
Measuring atmospheric pressure. Historians credit Evangelista Torricelli with inventing the mercury barometer in the early 1640s. A tube filled with mercury is sealed at the top and sits with the open end in a container of the same liquid that’s open to the air. You might think the liquid would run out of the tube into the container. It doesn’t, thanks to the atmosphere’s pressure.
Torricelli and others in the seventeenth century realized that air pressure pushes down on the liquid in the open container, pushing it up into the tube. If the liquid is water, as the first barometers were, the tube has to be at least 35 feet long to have empty space at the top. With mercury, which is much denser than water, a 34-inch tube works. This means the instrument can sit on a table or hang from a wall.
Air pressure measurements. In the United States you are most likely to see air pressure at the surface measured in inches of mercury. The National Weather Service uses inches of mercury in public forecasts and in reports of the altimeter setting that pilots use. The NWS also uses millibars for upper air pressures and for surface pressures in scientific reports.
Most of the rest of the world uses
hectopascals for both scientific and public reports of air pressure. Hectopascals and millibars are different names for the same figures. Both are direct measurements of pressure.
Meteorologists and other scientists began switching from describing atmospheric pressures in terms of inches or centimeters of mercury in the early twentieth century as they were making meteorology a mathematical science. A direct measurement of pressure such as millibars can be used in mathematical formulas describing the atmosphere. “Inches of mercury” is one of those antique terms that have hung on, like talking about an engine’s horsepower.
Different measures. Reporting the atmospheric pressure at a weather station is more complicated than reading the number indicated on a barometer. Here are the different kinds of surface air pressure that meteorologists use:
Station pressure. The reading directly from a barometer. It is not included in regular weather reports. For any station at an elevation higher than mean sea level, this reading will be lower than the ones reported.
Sea level pressure. Calculation of sea level pressure uses the current station pressure and temperature, as well as the station’s elevation. The observer assumes that the properties of the theoretical atmosphere extending down to sea level would be the same as at the station. Sea level pressure is used to draw weather maps with areas of high and low pressure. If station pressures were used for this, the lowest pressures would always be over the highest elevations.
Altimeter setting. This calculation is similar to the one used to calculate sea level pressure, but without using temperature. You can use your airplane’s altimeter when it’s on the ground to find the altimeter setting, if the altimeter is accurate. Just adjust the altimeter so the altitude that it reads is the elevation where the airplane is located. The number that shows up in the Kollsman window on the face of the altimeter is the altimeter setting for that location.


